Mycetoma Pathology

Prof. A.M. EL Hassan
Mycetoma Pathology
The Late
Prof. A.M. EL Hassan. MRCP, MRCPath, PhD, DCP
Emeritus Professor of Pathology.
Mycetoma Research Group.
Institute of Endemic Diseases.
University of Khartoum.

Prof EL Hassan seeing the first-ever patient at the Mycetoma Research Center’s new premises in 2008
Background
Mycetoma (maduromycosis) is a chronic granulomatous subcutaneous infection caused by actinomycetes (actinomycetoma) or by true fungi (eumycetoma). Clinically, the disease is characterised by swelling, deformity and sinuses in the affected part. Another characteristic feature of mycetoma is the formation of aggregates of the organism (grains) in the tissues, which are visible to the naked eye and are discharged through sinuses in the skin. The grains vary in colour, size and consistency depending on the causative agent. These features are helpful in making a tentative diagnosis of the causative organism.

A definitive identification of the agent is established by the histological examination of the grains, by culture or by serologic techniques. Both actinomycetoma and eumycetoma prevail in the Sudan. Eumycetoma due to Madurella mycetomatis is the commonest type. The actinomycetoma is commonly caused by Streptomyces somaliensis, Actinomadura madurae and Actinomadura pelletierii.
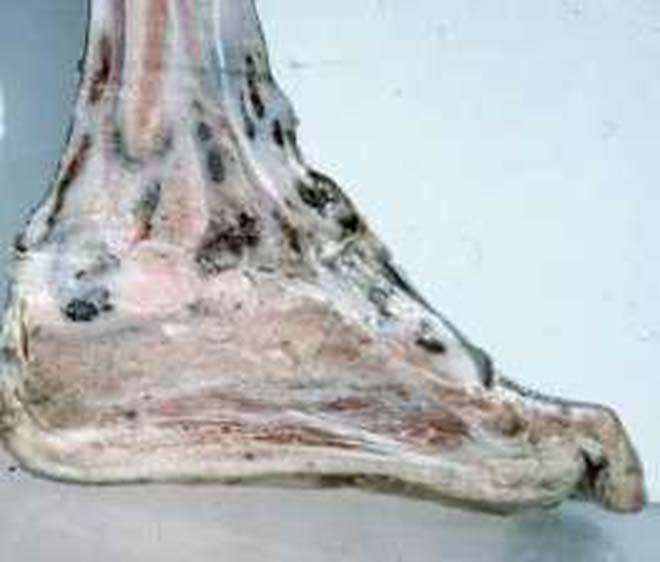
This article describes the pathology of the commonest types of mycetomata in the Sudan in surgical material referred to the Pathology Department, Faculty of Medicine, University of Khartoum.
- Madurella mycetomatis
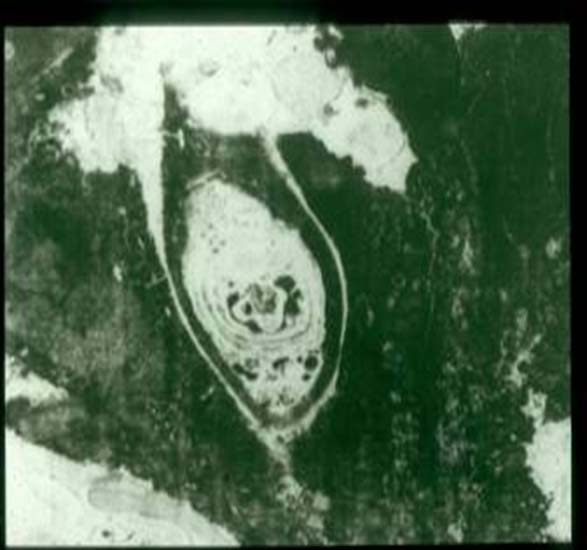
It is the most common causative agent of eumycetoma in the Sudan. In clinical material, the grains in the tissue are black and numerous. In stained sections, the grain is rounded, oval or trilobed. It has a more compact cortex, which is dark brown in colour due to pigment produced by the organism and has a lighter medulla. In some grains the division into cortex and medulla is not evident. The grain filaments are usually embedded in a hard brown cement matrix.

Two main morphological types of grains are identified. The filamentous, which is the commonest type, consists of brown septate and branched hyphae that may be slightly more swollen towards the periphery. In the cortex, the filaments are arranged radially while in the medulla, they tend to run multi-directionally. Round or oval cells, 7-15 um in diameter, are seen, particularly in the periphery.

The second type of grain is the vesicular one. It is less common than the filamentous and is composed of unusually large cells that look like vesicles. Both types of the grain can be found in the same lesion. Grains that are partially vesicular and partially filamentous are not uncommonly seen.
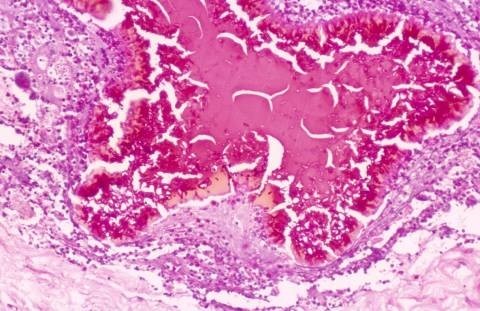
The surface of the grain may be scalloped, giving the periphery a hob-nail appearance and a brightly eosinophilic layer of fibrin–like material that sometimes covers the grain. This layer is of host origin and contains fibrin, immunoglobulins and complement. A similar material is seen around parasites as Hoepple phenomenon. Occasionally, a thin layer of haematoxophilic granular material is found on the surface of the grain. This material is Feulgen positive and is derived from the nuclei of disintegrating inflammatory cells surrounding the grains.
The inflammatory cellular reaction around the grain is variable. There are three types of tissue reactions. In type I, there is a zone of neutrophils in the vicinity of the grain. These are sometimes found within the grain substance, causing its disintegration. Some histocytes may also be seen among the neutrophils, but they are more numerous outside the neutrophil zone. Some of the histocytes have a foamy cytoplasm and give a positive reaction for fat and stain positive for CD 68 antigen. Capillaries, which are sometimes abundant, surround the neutrophil/ histiocyte zone, and they are occasionally surrounded by a layer of fibrin. Lymphocytes, plasma cells, fibroblasts and some macrophages are usually seen in this vascular zone. The lymphocytes and plasma cells increase in number towards the investing fibrous tissue in the periphery of the lesion.
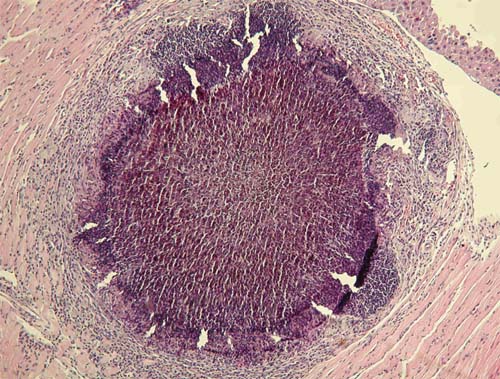
In type II reaction, the neutrophils largely disappear and are replaced by histocytes and multinucleated giant cells. Some of the latter contain fragments of grain or pigmented cement substance without any hyphae. The macrophages may contain a black pigment derived from the grain. At this stage, the grain itself is usually small and fragmented. This type of histocytes/giant cell reaction follows on the earlier neutrophil response, which causes fragmentation of the grain.

In the third type of reaction (Type III), the grain material has largely or completely disappeared, leaving a compact epithelioid granuloma with or without Langerhans giant cells. This, however, is an uncommon reaction and represents spontaneous regression in some grains.
The three types of tissue reaction may be found in the same lesion. Viable grains are nearly always present in biopsy material.

It is not known if spontaneous regression of all the grain ever occurs in mycetoma. The unique feature of M. mycetomatis is the formation of a capsule around the lesion. The lesion grows by expansile growth in the tissue plains.

In the bones, there is usually no capsule formation. The organism usually forms cavities that are filled with the grains. This gives the bone support and may explain the rarity of pathological fractures in mycetoma.
Ultrastructurally, the hyphal elements are spherical to elongated and are embedded in grain matrix. The hyphae are septate, and the cytoplasm may be densely ribosomal or disorganised. Some hyphae appear empty, being devoid of cytoplasm. Cytoplasmic organelles such as nuclei and mitochondria are not usually seen. Intra–hyphal re-growth is sometimes seen. The hyphal wall is often markedly thickened. This feature may be involved in the transformation of the fungus to the pathogenic state.
The pigmented substance surrounding the hyphae consists of amorphous electron-dense material and vesicles. The nature of the pigment is not known with certainty. Histologically, it resembles melanin, and it may be a fungus product. Sclerosis and melanisation of the host tissue are, in some manner, responsible for the formation of the cement substance.
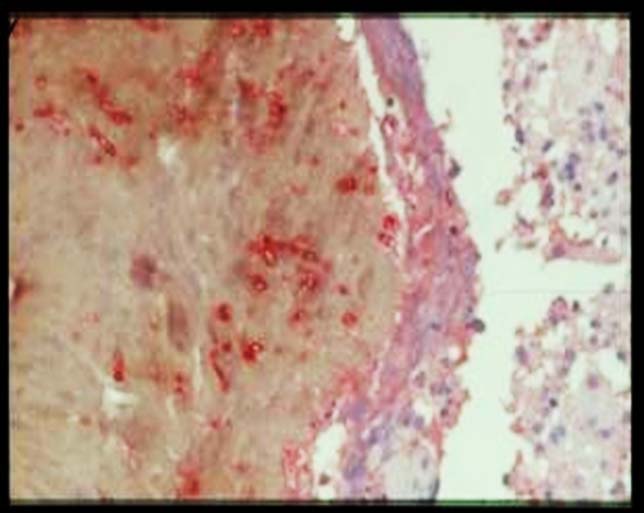
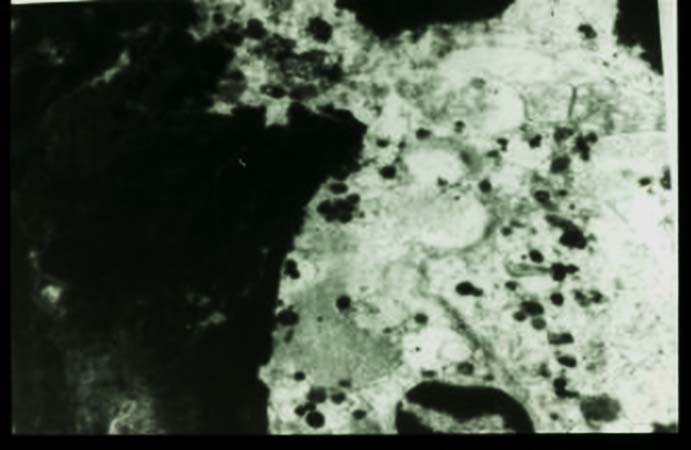
Ultrastructural studies of the host reaction show neutrophils adherent to the grain. The cytoplasm of the neutrophil is stretched over the grain, and the neutrophil granules are concentrated in the part of the cytoplasm adjacent to the grain. This is an immune adherence, which is mediated by immunoglobulins and is an example of antibody-dependent cell-mediated cytotoxicity. Immunoglobulin and complement can be demonstrated in the grain.
Bones are frequently involved in advanced mycetoma of the soft tissue. Occasionally, primary bone mycetoma is seen in the absence of soft tissue involvement. M. mycetomatis produces lytic lesions, which are large in size, few in number and have well-defined margins; this is well seen radiologically.
Streptomyces somaliensis
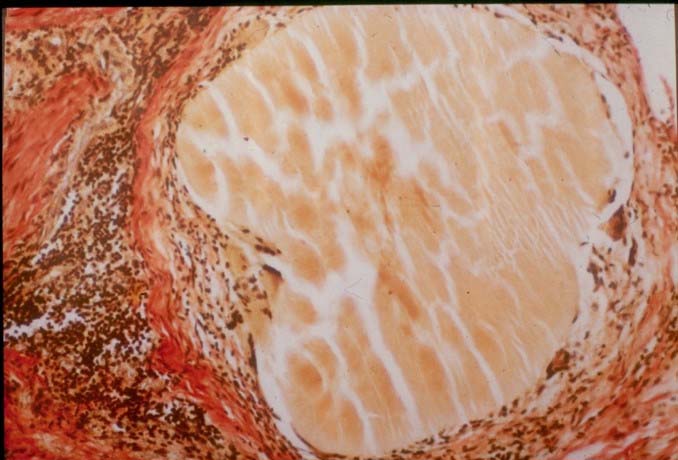
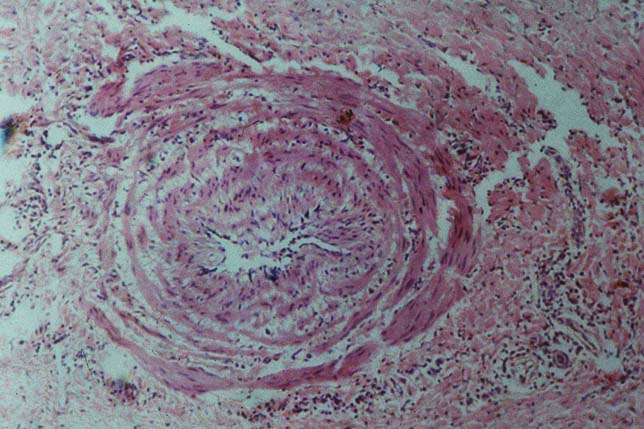
The grains are yellow in colour and hard in consistency. During surgery, it may be difficult to distinguish the grains from fat, which makes radical excision of the lesion difficult. This is especially so since the lesion is not encapsulated. In sections, the grain is rounded to oval, dense and homogenous. Characteristically, marks of the microtome knife are seen in the grain in the form of parallel cracks. The grain stains a light purple or pink colour in haematoxylin and eosin-stained sections. The grain varies in size from 30 to 200 um. Hyphal elements embedded in cement can be visualised by Gram stain.
The grain is surrounded by an intense neutrophil polymorphonuclear leucocyte infiltrate (Type I). Outside this zone, there is a vascular layer containing macrophages, lymphocytes, plasma cells and giant cells. The giant cells usually contain fragments of the grain. Some macrophages have a foamy cytoplasm. It looks as though the fragmentation of the grain induced by neutrophils is less severe than in M. mycetomatis. This may be due to the more compact and hard grains of Streptomyces somaliensis. Small grains surrounded by macrophages and giant cells (Type II) are occasionally seen, but pure epithelioid granuloma (Type III) apparently does not occur. Giant cells containing viable actinomycetes are believed to aid the spread of the organism in the tissue and to the regional lymph nodes. Despite the invasive nature of Streptomyces somaliensis and other actinomycetes, tendons and nerves are resistant to invasion.
Ultrastructurally, the grain consists of a heterogenous and amorphous matrix arranged in an irregular and reticulate structure surrounding electron lucent areas between 1 and 5 um. In some of these spaces, bacterial filaments are found. The organisms are usually unicellular and coccoid, and the cell wall is electron-dense.
Actinomadura pelletierii
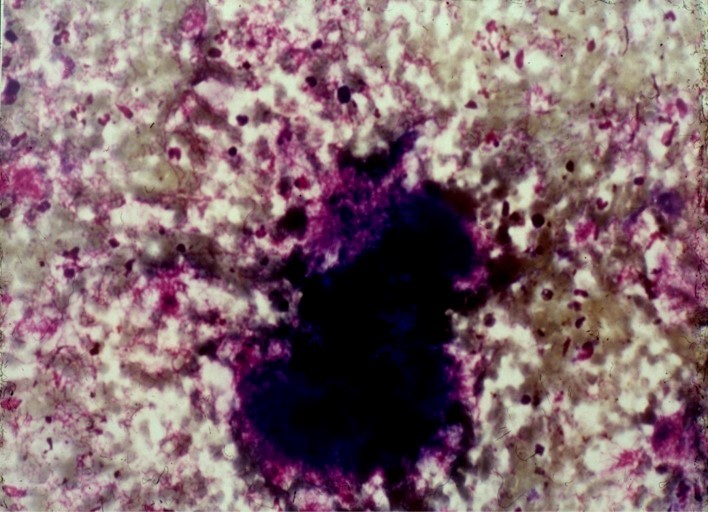
The grains in clinical material are tiny and red in colour. In section, the grain is rounded, oval or semilunar. It stains a purple colour, and compact hyphae give it the appearance of “Iron filings”. The periphery of the grain has a narrow, deeply eosinophilic band. The grain is usually surrounded by a zone of neutrophils, which causes fragmentation of the grain. The other layers are similar to those seen in Streptomyces somaliensis, but the giant cells are less conspicuous.
The ultrastructure of the grain is quite distinctive. The hyphae are septate, compact without cement substance and under low magnification the hyphae have a starry sky appearance because of the vacuoles in the hyphae. These are probably fixation artefacts. Neutrophils usually adhere to the grain and degranulate. Grain material is phagocytosed by the neutrophils and destroyed.
Actinomadura madurae
Macroscopically, the grains are yellow or white. They are difficult to distinguish from the surrounding fat. Histologically, the large grains have a characteristic variegated pattern. The periphery of the grain is dense, homogenous and deep purple, while the centre is less dense or even appears hollow. Not infrequently, the grain fragments into geometric fragments. The periphery shows a brightly eosinophilic material forming clubs. This material contains immunoglobulins. Smaller grains are more homogeneous and are difficult to distinguish from Actinomadura pelletierii.
However, even the small grains of Actinomadura madurae have a more deeply stained purple fringe, which is not seen in Actinomadura pelletierii. The inflammatory reaction is similar to that of Actinomadura pelletierii.

Vascular changes in mycetoma
The arteries and veins in the mycetoma lesion show hypertrophy of the muscles. The lumen is narrowed but is not occluded completely. Grain fragments are occasionally seen within the blood vessels. These may explain the rare haematogenous spread to distant sites.
Cytopathology of mycetoma
Fine needle aspiration cytology of mycetoma was described. Mycetoma has distinct cytological features characterised by the presence of polymorphous inflammatory cells consisting of neutrophils, lymphocytes, plasma cells, histocytes and foreign body giant cells. The causative organism is identified, in most cases, surrounded or/ and infiltrated by neutrophils. Maderulla mycetomatis grains are rounded or oval in shape. In Giemsa stained smears, they appear black with a green tinge of colour and brownish in H&E. Two types of Madurella mycetomatis grains could be identified in cytological smears, the solid granular one, which is the commonest and the vesicular. The septate hyphae are not identified in the first type as they are embedded in a hard brown cement matrix. The vesicular type consists of swollen fungal cells, seen as vesicles.
Actinomycetes grains are homogeneously eosinophilic in H&E. In Geimsa-stained smears, the grain appears homogeneously blue in the center while in the periphery it consists of fine granules and radiating pink filaments.
The Actinomadura pelletieri grain is more eosinophilic in H&E than Streptomyces somaliensis, and it is semilunar in shape, as seen in histology. To distinguish between actinomycetoma and eumycetoma, fine needle aspiration cytology was found to be as accurate as histopathology when grains are present. The cytological features of mycetoma correlate well with the histologic ones.
The technique is simple, quick and cheap. It can be introduced for routine diagnosis of mycetoma, epidemiological surveys, and culture sample collection.
Bony changes in mycetoma
Bones are frequently involved in advanced mycetoma of the soft tissue. Occasionally primary bone mycetoma in the absence of soft tissue involvement is seen. Madurella mycetomatis produces lytic lesions that are large in size, few in number, and have well-defined margins.
Actinomycetoma destroys bone and also evokes new bone formation. The cavities produced are usually smaller in size, numerous and have no definite margins. A periosteal reaction with new bone formation, Codman’s triangle and sun-ray appearance may be seen in radiographs. These features, along with Codaman’s triangle, simulate the radiographic changes in osteogenic sarcoma.

Lymph nodes in mycetoma
Lymph nodes draining a mycetoma focus are frequently enlarged. Most of the nodes show only reactive hyperplasia with an intense plasma cell infiltration. The plasma cells often contain Russell bodies. These changes may be due to antigens reaching the nodes from grains at the primary site. Secondary bacterial infection of the mycetoma lesion may also be a factor. Occasionally, the organism metastasises to the nodes, causing lymphadenitis with sinuses discharging grains. This is more frequent with actinomycetoma than with eumycetoma. Haemosiderin deposits are not infrequently found in the nodes, even in the absence of grains. The haemosiderin is derived from the granulation tissue in the peripheral lesion draining into the lymph nodes. Melanin is also found in the node and may be confused with the pigment of Madurella mycetomatis.
Further reading:
- EL Hassan AM, Fahal AH, EL Hag IA, Khalil EAG, The pathology of mycetoma: Light microscopic and ultrastructural features. Sudan Med J. 1994; 32: 23-45.
- Fahal AH, EL Toum EA, EL Hassan AM, Gumaa SA, Mahgoub ES, A preliminary study on the ultrastructure of Actinomadura pelletierii and its host tissue reaction. J Med Vet Mycol. 1994; 32: 343-348.
- Mahgoub ES, Murray TG. Mycetoma William Heineman, Medical Books, London.
- Fahal AH, el Toum EA, el Hassan AM, Mahgoub ES, Gumaa SA. The host tissue reaction to Madurella mycetomatis: new classification. J Med Vet Mycol. 1995 Jan-Feb;33(1):15-7. PMID: 7650573.
- EL Hag IA, Fahal AH, Khalil EAG, Fine needle aspiration cytology of mycetoma. Acta Cytologica. 1996; 40(3): 461-464.
- Fahal AH, EL Hag IA, Gadir AFA, EL Lider AR, EL Hassan AM, The blood supply and vasculature in mycetoma. J Med Vet Mycol. 1997; 35:101-106.
- Yousif BM, Fahal AH, Shakir MY. A new technique for the diagnosis of mycetoma using fixed blocks of aspirated material. Trans R Soc Trop Med Hyg. 2010 Jan;104(1):6-9. doi: 10.1016/j.trstmh.2009.06.015. Epub 2009 Aug 22. PMID: 19700179.
