MRC- In Vitro Susceptibility Integrated Unit
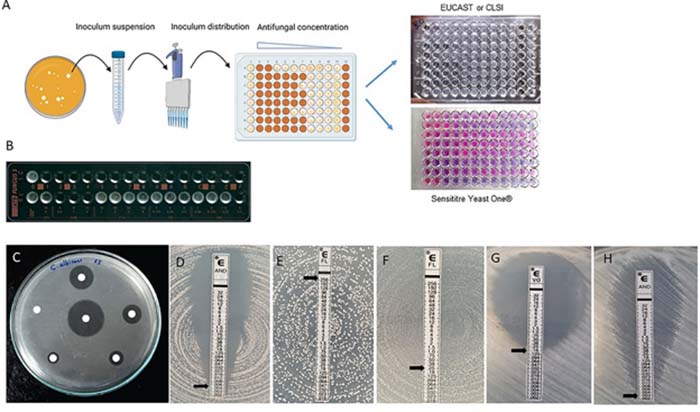
The In Vitro Susceptibility Integrated Unit within the Mycetoma Research Center (MRC) is dedicated to the identification and combatting of antimicrobial-resistant agents associated with mycetoma. Established to address the growing challenge of antimicrobial resistance in mycetoma-causing organisms, this unit adopts a comprehensive approach integrating in vitro susceptibility testing methods.
The primary objective of the Unit is to assess the susceptibility of mycetoma-causing agents to various antimicrobial agents through advanced in vitro techniques. By utilising cutting-edge methodologies, the unit aims to understand the resistance profiles of these organisms, providing crucial insights for developing effective treatment strategies.
This specialised unit operates within the framework of the Mycetoma Research Center and plays a critical role in contributing to the global effort to combat antimicrobial resistance. It actively engages in research and development initiatives, collaborating with multidisciplinary teams to enhance our understanding of the dynamics of resistance mechanisms and identify novel interventions.
As part of its integrated approach, the Unit collaborates with other units within the MRC, fostering a synergistic environment for comprehensive research and development. Through its endeavours, the unit strives to advance the identification and management of antimicrobial-resistant mycetoma agents, ultimately contributing to global efforts to mitigate the impact of antimicrobial resistance on public health.
The unit operates with the objectives to:
- Devise cutting-edge, user-friendly, and cost-effective protocols tailored for environments where mycetoma infections are prevalent.
- Innovate an alternative approach for generating hyphal suspension in eumycetoma cases, and validate the use of this inoculum in various in vitro susceptibility testing techniques, including broth microdilution, the E test, disc diffusion, and VIPcheck. This validation is conducted in comparison to the modified CLSI broth microdilution (BMD) technique developed in 2003.
